UK New Approach Methodologies Roadmap (2023) Draft Version 3
On this page
Skip the menu of subheadings on this page.This is a paper for discussion.
This does not represent the views of the Committee and should not be cited.
Introduction
- Advances in biology, computer science and other related fields are paving the way for major improvements in how we evaluate environmental and public health risks posed by potentially toxic chemicals.
- Some of the current challenges faced by UK chemical risk assessors are: the large number of (groups of) chemicals that require assessment, lack of toxicological data on these chemicals and the speed, cost and ethical and moral considerations of traditional testing methods. One of the major recent scientific advancements is the development of New Approach Methodologies (NAMs) including but not limited to high throughput screening and other in vitro assays, omics and in silico computer modelling strategies (e.g. Artificial Intelligence (AI) and machine learning) for the evaluation of hazard and exposure. This also advocates the Replacement, Reduction and Refinement (3Rs) approach to animal testing.
- NAMs are gaining traction as a systematic approach to support informed decision making in chemical risk assessment.
- The future of food safety assessment of chemicals depends on adaptability and flexibility whilst using the best scientific methodologies and strategies available in order to respond to the accelerating developments in science and technology. The vision is to be able to predict risk more accurately, rapidly and efficiently. In addition, it is about using the best science available and integrating innovative technologies into the chemical risk assessment process. This will be fundamental in the future for human and environmental safety.
- The UK Food Standards Agency (FSA) and Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) have been considering New Approach Methodologies (NAMs) to understand the best scientific methodologies available for use in the risk assessment of chemicals and to consider how these can be incorporated and accepted in a regulatory context.
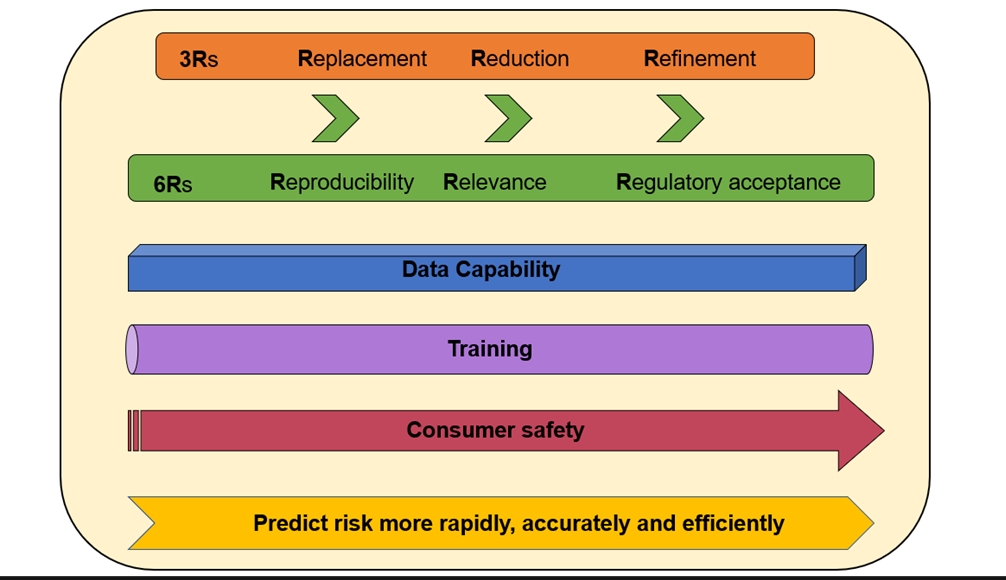
- In order to achieve this, the FSA and COT are developing a UK New Approach Methodologies (NAMs) roadmap towards acceptance and integration of NAMs including predictive toxicology methods using computer modelling into safety and risk assessments for regulatory decision making. This will not only require the historic 3Rs approach (i.e. replacement, reduction and refinement of animal experiments) but the expansion to the 6R principle: (to also include) reproducibility, relevance, and regulatory acceptance.
- The COT have reviewed a scoping paper (TOX/2019/70) and the COT and FSA have organised three workshops with worldwide participation with attendees from industry, academia and regulatory agencies covering a wide range of topics which included but are not limited to physiologically based pharmacokinetic (PBPK) modelling and simulation, artificial intelligence tools, dose response, legal and sociotechnological factors.
- The first draft of the UK roadmap (TOX/2021/31) was presented to the COT in 2021 (COT July Final Minutes 2021).
- The COT FSA workshop Paving the way for a UK Roadmap: Development, Validation and Regulatory Acceptance of New Approach Methodologies (NAMs) in Chemical Risk Assessment held in October 2021 (COT October Final Minutes 2021) was to receive insights, comments and input from a wide variety of stakeholders as well as industry, academia and government, on the roadmap version 2 so that they could develop and produce a useful and engaging document that is beneficial to more than just the FSA and COT. The outcomes of the workshop were used to further develop the next version of the UK NAMs Roadmap and promote collaboration across regulatory agencies, academia and industry.
- After presenting at various international conferences, meetings and workshops, Members are asked to note the recent updated draft version of UK NAMs roadmap attached at Annex A which incorporates the feedback received. This includes data integrity and capability, training and the integrated transition into acceptance.
- This paper is presented largely for information, but Members are welcome to comment or contact the Secretariat if they have any detailed comments.
Secretariat
May 2023
Paving the way for a UK Roadmap:
Development, Endorsement and Regulatory Acceptance of New Approach Methodologies (NAMs) in Chemical Risk Assessment and Beyond.
Contents Page Number
|
Executive summary |
3 |
|
Introduction & Background |
4 |
|
Chemical Landscape |
4 |
|
What are NAMs and why is there a drive in the regulatory context? |
4 |
|
Who are the UK FSA and the COT? |
6 |
|
Why are the FSA and COT taking a lead on this? |
7 |
|
FSA Digital Vision |
7 |
|
Food Security is a top priority: The Government Food Strategy X FSA Strategy = |
7 |
|
Food is safe + Food you can trust |
|
|
Regulatory Acceptance of NAMs |
7 |
|
UK Government cross cutting themes on NAMS, data and emerging technologies |
8 |
|
Future Government cross themes |
8 |
|
What have the FSA / COT done so far? |
10 |
|
The proposal: How does the FSA plan to integrate NAMs in the regulatory space? |
13 |
|
Pillar I: Development |
14 |
|
Pillar II: Endorsement |
15 |
|
Pillar III: Regulatory Acceptance |
15 |
|
The 7 Steps to Integration & Acceptance |
19 |
|
Stepping and (Mile)Stones - How the FSA got here |
23 |
|
Future Visions: The new normal chemical landscape |
25 |
|
References |
26 |
|
Abbreviations |
29 |
|
Technical information |
30 |
Executive summary
Advances in biology, computer science and other related fields are paving the way for major improvements in how we evaluate environmental and public health risks posed by potentially toxic chemicals. The combined advances in discovery and clinical sciences, data science and technology have resulted in toxicity testing which has reached a pivotal transformation point known as the 4th industrial revolution (4IR). One of the major recent scientific advancements is the development of new approach methodologies (NAMs) which includes but not limited to computer modelling strategies for the evaluation of hazard and exposure whilst championing the Replacement, Reduction and Refinement (3Rs) of animals, approach.
The volume of data produced in the world is growing ever more rapidly, from 33 zettabytes in 2018 to an expected 175 zettabytes in 2025 (IDC, 2018) (Food Systems). The Department for Business, Energy and Industrial Strategy (BEIS) white paper on Regulation for the Fourth Industrial Revolution notes that changes in technology are occurring at a "scale, speed and complexity that is unprecedented". The use of such technologies can help improve regulatory processes in several ways such as to improve the efficiency of data collection and to exploit data already held by agencies to support better analysis and risk assessment (BEIS Report-The use of emerging technologies for regulation).
The future of food safety assessment of chemicals depends on our adaptability and flexibility whilst using the best scientific methodologies and strategies available in order to respond to the accelerating developments in science and technology.
The vision is to be able to predict risk more rapidly, accurately and efficiently.
For regulatory agencies to incorporate and implement these new predictive capabilities brings both challenges and opportunities. Moving from research to risk assessment to regulatory setting and beyond, there must be suitable validation and acceptance of these new and emerging technologies.
Using a process via an evidence driven approach to address the data gaps in the risk assessment process will facilitate the acceptance and validity of these NAMs as well as pave the way for alternative methods. Integration of these technologies as part of the risk assessment process will be fundamental in the future of human and environmental safety.
In order to achieve this, the Food Standards Agency (FSA) and Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) have developed a UK roadmap towards acceptance and integration of these new approach methodologies including predictive toxicology methods using computer modelling into safety and risk assessments for regulatory decision making.
This will not only require the historic 3Rs approach (i.e. replacement, reduction and refinement of animal experiments) and the expansion to the 6R principle: (to also include) reproducibility, relevance, and regulatory acceptance.
Introduction & Background
Chemical Landscape
“The Toxicity Data Landscape for Chemicals” paper (Judson et al., 2009) reported that 28 million chemicals had been discovered. Only three million had been tested on animals and some of these also tested in human studies. Approximately, a further one million had some in vitro/in silico screening assay data only. By 2020, over 350,000 chemicals and mixtures of chemicals had been registered for production and use worldwide. The identities of many chemicals remain publicly unknown because they are claimed as confidential (over 50,000) or ambiguously described (up to 70,000) (Wang et al., 2020).
It is understandable that with those numbers, finding an accurate and optimized model poses a big challenge, not least for example some of the environmental chemicals that don’t necessarily come with a toxicological package. However, by combining data from traditional methodologies with data from these new emerging technologies we will be able to predict chemical risk more accurately, rapidly and efficiently.
Hopefully this will also be an opportunity to engage cross cutting themes, continue to collaborate with: the National Centre for the Replacement, Refinement and Reduction of Animal Research (NC3Rs); Partnership for the Assessment of Risks from Chemicals (PARC) and feed into other projects e.g., Genome UK: the future of healthcare (2020); and Accelerating the Pace of Chemical Risk Assessment (APCRA), as well as collaborations across academia, industry and beyond.
Current Chemical Challenges
- Too many chemicals to assess.
- Traditional methods can be slow, costly and have ethical considerations.
- Adopting the principles of the 3Rs (i.e. Replacement, Reduction and Refinement of animal experiments).
- Not enough data on compound/compounds being assessed.
Opportunities
- Adopt new approach methodologies (NAMs) to predict risk more rapidly, accurately, and efficiently towards optimum consumer safety.
What are NAMs and why is there a drive in the regulatory context?
Advances in biology, computer science and other related fields are paving the way for major improvements in how we evaluate environmental and public health risks posed by potentially toxic chemicals. The combined advances in discovery and clinical sciences, data science and technology have resulted in toxicity testing which has reached a pivotal transformation point known as the 4th industrial revolution (4IR). One of the major recent scientific advancements is the development of NAMs including but not limited to high throughput screening, organ on chips, omics and in silico computer modelling strategies (e.g. Artificial Intelligence (AI) and machine learning) for the evaluation of hazard and exposure. This also advocates the Replacement, Reduction and Refinement (3Rs) of animals, approach (Hartung, 2010) proposed 50 years ago by Russell and Burch (1959). Our collective definition of the usage of NAMs is an assessment which combines and integrates data from traditional methodologies with data from these new emerging technologies.
What is being done?
To keep pace with the digital evolution, we aim to use the latest technology and best available scientific methodologies to incorporate additional tools into our regulatory risk assessment process to evaluate safety in food, consumer products and the environment more efficiently and without compromising quality.
Why?
This will enable us to provide improved risk assessments of chemicals for which there are currently no, or very few data, and therefore increase consumer safety.
NAMs will help us to predict risk more rapidly, accurately, and efficiently.
Who are the UK FSA and the COT?
The UK FSA is an independent Government department working across England, Wales, and Northern Ireland to protect public dietary health and consumers' wider interests in food. The FSA uses expertise and influence so that people can trust that the food they consume is safe and is what it says it is. The Science, Evidence and Research Division (SERD) of the FSA provides strategic analysis, insight, and evidence across the FSA’s remit to underpin the development of policies, guidance, and advice on food safety.
The COT assesses chemicals for their potential to harm human health. Scientific evaluations are carried out at the request of the FSA, Department of Health and Social Care (DHSC), UK Health Security Agency (UKHSA), and other government departments and regulatory authorities.
Why are the FSA and COT taking a lead on this?
The FSA responds to food incidents and it is imperative that risk assessments on the safety of a chemical are provided. The data used can only be what is available and sometimes there is very little, or no, toxicological information for a given chemical.
For these chemicals, the use of NAMs will provide a more indicative level of risk and therefore greater confidence in risk assessments that individual compounds can be assessed. This will be fundamental in risk assessment scenarios where limited to no information is available on the toxicity of a chemical.
The FSA and COT have been reviewing the use of NAMs to scope what methods are out there, in order for the best methodologies to be used in risk assessment and understand how these can be incorporated in a regulatory context (Environmental, health and safety alternative testing strategies: Development of methods for potency estimation TOX/2019/70). There is a need for integration of several methodologies which will form part of the integrated approaches to testing and assessment (IATAs). In order to build confidence in what we are trying to achieve this roadmap will not only require the historic 3Rs approach (i.e. replacement, reduction and refinement of animal experiments) but also the expansion to the 6Rs principle: (to also include) reproducibility, relevance, and regulatory acceptance. Furthermore, data capability expansion will need to be taken into consideration.
FSA Digital Vision
In the FSA Chief Scientist Data Science Report (2017), it stated that “Big data and data science bring relatively new tools and techniques to Government analytics”.
In the Science Council Working Group on Data Usage and Digital Technology Final Report to the FSA it stated in one of the recommendations: “Encourage the development of data capabilities and skills across the FSA staff base therefore, we want to embrace the new technologies and enhance capabilities for our staff.”
Food Security is a top priority: The Government Food Strategy X FSA Strategy = Food is safe + Food you can trust
In the Government food strategy food security is one of the main pillars and using NAMs will enable regulators to make sure food is safe, which underpins the FSA strategy in which the fundamental mission is: food you can trust.
Regulatory Acceptance of NAMs
NAMs and IATAs are currently rarely accepted by regulatory bodies. However, it is clear that we are now at a pivotal point where integration of such (methods/techniques) will be fundamental in more efficient, ethical and rapid risk assessment. The key question is how these approaches can be facilitated in a regulatory setting using the supporting technologies available. The use of these methods, through various case studies, as a ‘proof of principle’ concept is becoming apparent.
Worldwide perspectives on emerging technologies
The focus of the 7th annual Global Summit on Regulatory Science (GSRS17) was Emerging Technologies for Food and Drug Safety and the summit publication stated that “Moving forward toward greater integration of emerging data and novel methodologies for chemicals risk assessment will need continuous efforts on capacity building” (Slikkler et al., 2018).
Furthermore, in the recent EU Farm to Fork strategy and the EU Green Deal Food 2030 Pathways for Action (Food Systems and Data 2020) it states: “to valorise emerging technologies, tools, standards and infrastructure for use in food systems”.
The Royal Society of Chemistry recently published a Drivers and scope for a UK chemicals framework (2021) which recommends under regulation: “Be a world leader in the development of New Approach Methods (NAMs) for safety evaluation without the use of animals and devise new risk assessment frameworks to support decision-making using NAMs”.
The future direction of safety assessment science will depend heavily on the evolution of the regulatory landscape. A key challenge, though, is whether the regulatory framework can keep pace with the increasing speed of scientific and technological developments (Worth et al., 2019).
Food authorities should strive to incorporate the best scientific methods available (Kavlock et al., 2018) into chemical risk assessment. The future of food safety assessment depends on adaptability, flexibility and revolutionary principles in order to respond to the accelerating developments in science and technology.
This implies that close collaboration will be needed between chemists, toxicologists, informaticians, risk assessors and others to develop, maintain and utilise appropriate models. Not only must the different disciplines come together, but also those scientists from industry, academia and regulatory agencies must recognise the commonalities (Cronin et al., 2019). The challenge is to respond to the growing need for adaptable, flexible and even bespoke computational workflows that meet the demands of industry and regulators, by exploiting the emerging methodologies of Tox21 and risk assessment.
UK Government cross cutting themes on NAMS, data and emerging technologies
There are numerous examples of work happening in government and beyond, currently surrounding NAMs and data capabilities needs. Therefore this roadmap fits in well, feeding and integrating valuable information into the wider remit to progress scientific and technical capabilities towards benefiting society and protection of human safety.
- The Alliance for Human Relevant Science formed a new Parliamentary group which calls for human relevant science moonshot. The purpose is for MPs and Peers of all parties to accelerate the development and uptake of human relevant life sciences in the UK on medicines development. The report Bringing back the human: transitioning from animal research to human relevant science in the UK highlights its desire to use NAMs, and under regulatory recommendations suggested a NAMs MHRA working group. The FSA have established a Cross-Whitehall group in which various government departments and agencies are involved.
- In the ‘Rebuilding a Resilient Britain’ programme one of the departmental Areas of Research Interest (ARIs) marked as a priority was “data science and digital technologies” in the “Changing Systems” theme.
- In the “The Integrated Review of Security, Defence, Development and Foreign Policy Global Britain in a competitive age” it requests a collaborate-access framework to guide government activity in priority areas of Science & Technology (such as AI, quantum technologies and engineering biology) which has the potential to unlock a step-change in wide ranging applications, which include chemicals.
- The volume of data produced in the world is growing ever more rapidly, from 33 zettabytes in 2018 to an expected 175 zettabytes in 2025 (IDC, 2018) (Food Systems and Data). The Department for Business, Energy and Industrial Strategy (BEIS) white paper on Regulation for the Fourth Industrial Revolution notes that changes in technology are occurring at a "scale, speed and complexity that is unprecedented". The use of such technologies can help improve regulatory processes in several ways. These include the improvement of the efficiency of data collection and to better exploit data already held by agencies to support improved analysis and risk assessment.
- DEFRA’s science research program on integrating systems thinking approach includes Food systems in which NAMs, when used in the food system, could compliment in a regulatory context.
- Life Science Vision 2021 states that Life Sciences will be one of the great drivers of growth in the twenty first century. Through innovation and technological advances, we will diagnose, treat, cure and prevent a much wider range of disease than is currently possible. One of the strategic goals is in the genomics field to harness the UK’s prior investments to fully integrate genomics into health service delivery through the Genomic Medicine Service, and deliver significant advancements in the understanding, diagnosis, and treatment of disease. In addition, health data will be used in a secure and transparent manner, harness the NHS’s unique health data to understand and tackle population health challenges, and drive advances in Life Science research and innovation. NAMs includes omics approaches and other tools that are also used in the health setting. Therefore, the new approaches and technologies that are developed can be shared across different settings and used towards improving knowledge and harnessing powerful collaborations.
- This UK COT FSA NAMs roadmap would complement the already existing UKRI Non-animal technologies in the UK: a roadmap, strategy and vision (2015) which recommends a need for early engagement with regulators to ensure that non-animal technologies can be used in regulatory risk assessments.
- The COT FSA NAMs roadmap will also will also help support various research strategies and priorities in the new UKRI strategy 2022 to 2027 such as Priority 5.2 in the UKRI Strategy: Harness the opportunities from tomorrow’s technologies, AI, digital and advanced computing. This supports the “development and use of cutting-edge tools, technologies and infrastructures– and in particular leverage the rapid advances in digital, data driven and computational approaches – that enable researchers and innovators to push boundaries”
- DHSC Better, broader, safer: using health data for research and analysis independent report states that its aims are: to facilitate substantially wider access to data; facilitate modern open working methods; and create a rapid explosion in the efficiency, openness, and quality of analytic work. NAMs can leverage the process of the harmonisation of a wide variety of data to predict human relevant data safety predictions including using AI and machine learning tools.
- The COT FSA NAMs roadmap will cross pollinate with government activity and The UK’s National AI Strategy the vision of which is to remain an AI and science superpower, fit for the next decade.
Future Government cross themes
Looking ahead this can form part of the UK government science superpower agenda. Examples include Advanced Research and Invention Agency (ARIA) which will form a critical part of the UK government’s science and research agenda. ARIA will focus on projects with the potential to produce transformative technological change, or a paradigm shift in an area of science. NAMs can form part of a paradigm shift in how we predict risk and assess the safety of chemicals.
The other example is quantum biology. Joint statement of the United Kingdom of Great Britain and Northern Ireland and the United States of America on cooperation in quantum information sciences and technologies recognised that quantum information science and technology (QIST) will explore new ways to enable enhanced acquisition, transmission and processing of information and could lead to the development of exponentially more powerful computers, novel communication networks, and more precise and accurate sensors. NAMs require enhanced data capabilities which will require powerful computers.
Finally, a recent UK Science and Technology Framework policy paper identified AI as one of the five critical technologies most critical to the UK and to take advantage of international collaborations so that we are influential in shaping the global landscape, embedding our values into technology including to lead international efforts to shape standards and regulations for critical technologies. It further outlined the need to have increased infrastructure capacity to deliver science and technology ambitions and promote data as an enabler, something the NAMs will be championing.
What have the FSA/COT done so far?
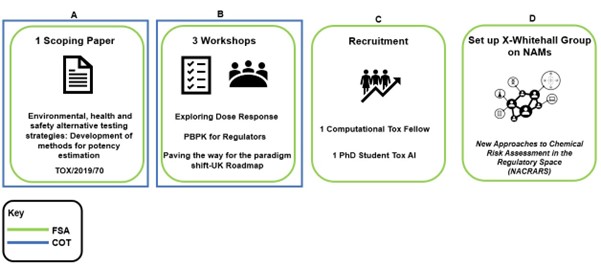
Figure 1. Diagram of what the FSA and COT have done so far in the NAMs space.
(A) Scoping paper on NAMs (B) 3 international workshops (C) recruited a computational toxicology fellow in Advanced in silico methods of assessing toxicological risk and a PhD Student on artificial intelligence tools to predict chemical risk (D) Set up a Cross Whitehall group on NAMs.
The scoping paper “Environmental, health and safety alternative testing strategies: Development of methods for potency estimation” (TOX/2019/70) was reviewed by the COT in December 2019. The COT were provided with a concise review of currently available methods, which included databases, different kinds of quantitative structure activity relationships (QSAR) methods, adverse outcome pathways (AOPs), High Throughput Screening (HTS), read-across models, molecular modelling approaches, machine learning, data mining, network analysis tools, and data analysis tools using artificial intelligence (AI).
The full workshop reports will be made available through the COT website once published (currently under reserved business). In 2021, the FSA recruited a computational toxicology postdoctoral Fellow at the University of Birmingham and a PhD Student at King’s College London as part of their Interdisciplinary Doctoral Program (LIDo-TOX AI). The fellow and PhD student have been working alongside other Government Departments to understand how NAMs will improve indicative levels of safety in chemical risk assessment. In addition, these new partnerships have helped with networking, research collaboration, training opportunities and other activities in this area. Some of the initial work was presented in 2022 to the COT (COT October 2022 Final Minutes) and we will continue to report on the projects. In 2022, the FSA created a Cross Whitehall steering group: New Approaches to Chemical Risk Assessment in the Regulatory Space (NACRARS). The role of the group is to encourage discussion and partnerships that will be instrumental in creating confidence and reliance in the use of NAMs in chemical risk assessment more widely.
In addition, this work will complement the report produced by Synthesis and Integration of Epidemiological and Toxicological Evidence Subgroup (SETE) of the COT and Committee on Carcinogenicity (COC) which was set up in 2019. Its aim was to review the approaches for synthesising and integrating epidemiological and toxicological evidence that are used by the COT and COC in chemical risk assessments and to provide a pragmatic guidance and transparent reflection of how the COT and COC review data.
Exploring Dose Response (EDR) Workshop Summary
The UK FSA and the COT held an “Exploring Dose Response” workshop (March 2020) in a multidisciplinary setting which included regulatory agencies, government bodies, academia and industry. The workshop provided a platform from which to address and enable expert discussions on the latest in silico prediction models, new approach methodologies (NAMs), physiologically based pharmacokinetics (PBPK) models, future methodologies, integrated approaches to testing and assessment (IATA) as well as methodology validation. Through case studies (including plastic particles, polymers, tropane alkaloids, selective androgen receptor modulators), the workshop outlined and explored an approach that is fit for purpose when applied to human health risk assessment in the context of future food safety assessment. Furthermore, possible future research, to establish points of departure (PODs) using non-animal alternative models and to improve the use of exposure metrics in risk assessment, was discussed.
Overall conclusions were as follows:
a) The use of pragmatic guidelines/framework for incorporating these models into risk assessment. Using case studies, such as those outlined in the workshop, towards applicability and gaining confidence in the models.
b) Human biomonitoring data will be key to identify realistic snapshots of exposure scenarios as well as big data which need to be linked to human clinical data.
c) Exposure data and exposure science will be key in developing in silico models in risk assessment and to explore the use of exposomics.
d) There should be transparency throughout the process i.e. consumer facing engagement on NAMs.
e) There should be planning, to take these new methods forward using social sciences research and technical research for integration.
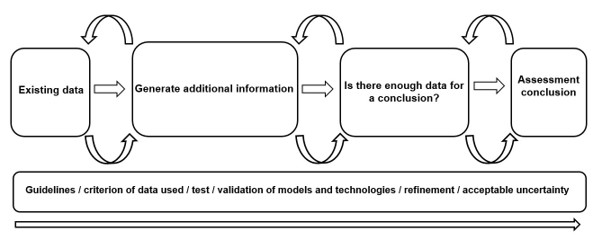
Ultimately, it was collectively agreed that integration of these new technologies as part of our risk assessment methodologies, with a validation process throughout, will be key in the acceptance of the models (by regulatory bodies) and will be fundamental in the future of human and environmental safety (Figure 2).
Figure 2. Concluding workflow for integration of NAMs in regulatory risk assessment from the EDR workshop.
PBPK for Regulators Workshop Summary
The UK FSA and the COT held a “PBPK for Regulators” workshop (December 2020) in a multidisciplinary setting with delegates from regulatory agencies, government bodies, academia and industry. The workshop provided a platform to enable expert discussions and presentations on the application of PBPK to human health risk assessment in a regulatory context as well as potential future research.
Main overarching conclusions of the PBPK workshop:
a) PBPK modelling tools are applicable in the explored areas of use, and there is some expertise available for their utilisation.
b) PBPK modelling offers opportunities from which to address questions for compounds that are otherwise not solvable.
c) Widespread acceptance amongst regulatory bodies appears to be limited by lack of available in-house expertise.
d) Familiarisation using real world case studies would help in developing more experts in the field and increasing acceptance.
e) In a regulatory context, establishing fitness for purpose for the use of PBPK models require multi-partite discussion and harmonised guidance.
f) Finally, PBPK modelling is part of the wider “new approach methodologies” for risk assessment.
Paving the way for a UK Roadmap-Development, Validation and Acceptance of New Approach Methodologies Workshop summary
The UK FSA and the COT held a workshop that took place online over 2 days in October 2021. It had participation from industry, academics and regulators. The aim of the workshop was to receive insights, comments and input from a wide variety of stakeholders and industry, academia and government, on the roadmap so that it can be developed and a useful and engaging document produced, that is beneficial to more than just the FSA and COT. This included a range of scientists, policy and lawyers, and working in the international space and engaging with the public. Furthermore, there were discussions on what is holding the progress back including a range of areas such as law, economics, socio-technical barriers and regulatory frameworks.
The proposal: How does the FSA plan to integrate NAMs in the regulatory space?
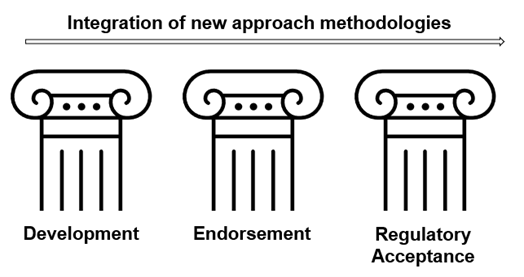
The pillars of the NAMs integration approach in the regulatory space will be: Development, Endorsement and Regulatory Acceptance.
Pillar I: Development Formulating the problem space
It is well known that designing and structuring your problem space (Goel and Pirolli,
1992) will be fundamental in its outputs (Newell et al., 1993) and success (Goel and Pirolli, 1989). The science of design consists of the use of efficient, available computational techniques (modelling) for finding the best course of action to respond to real situations, or reasonable approximations of real situations (Simon, 1981). Problem formulation is a systematic approach that identifies all factors critical to a risk assessment (Solomon et al., 2016) and will be key to the chemical risk assessment process.
Figure 3. Landscape to integrate NAMs in the regulatory space.
The FSA plans to use problem exploration techniques which includes interactive sessions at FSA-COT-led workshops, to discuss the use of NAMs in risk assessment and define the questions that need to be answered in order to use NAMs in regulatory chemical risk assessment.
|
Exploration |
| Identification |
| Allocation |
| Adoption |
| Integration |
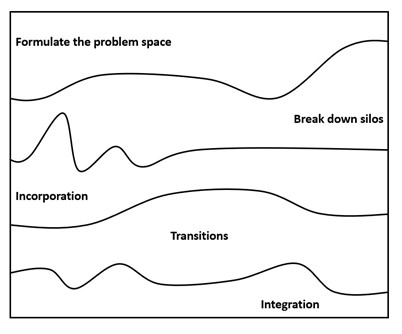
Figure 4. Formulating the problem space for project outputs.
Pillar II: Endorsement
Breaking Down the Silos: Drivers and Obstacles
The Malloy et al. (2017) paper recommends supporting trans-sector and transdisciplinary efforts to integrate predictive toxicology “Use existing efforts to bring together regulators, industry, civil society, and academics to agree on testing protocols for nanotechnologies as a model that then could be adopted in other fields”.
Despite advances, the scope and pace of adoption of NAMs, including toxicogenomics tools and data sets in chemical risk assessment, have generally, not met the ambitious expectations of their advocates (Pain et al., 2020).
Regulatory uptake of NAMs has been hesitant and despite notable improvements and new applications, some of the major obstacles remain, such as unconnected silos, interpretation complexities and acceptance of validation by regulators.
Pillar III: Regulatory Acceptance
Incorporation, Adopters of change, Data to Deployment
The incorporation and implementation of these new predictive capabilities by regulatory agencies brings both challenges and opportunities.
Moving from research to risk assessment to regulatory setting and beyond, the validation and acceptance of these new emerging technologies must be ensured. The FSA plan to learn from other regulatory agencies and other conceptual frameworks (e.g. IPBES Conceptual Framework (Diaz et al., 2015) i.e. to structure the syntheses that will inform policy, and to improve comparability across various assessments carried out at different spatial scales, on different themes, and in different regions) in different settings.
NAMs are gaining traction as a systematic approach (Sturla et al., 2014) to support informed decisions on chemical risk assessment. Adapting how we assess risk will be, and has always been, a challenge, but big changes have, and can, occur through innovation that facilitates and accelerates adaptation (Rogers, 2003). In Attwell’s 1992 paper, it was concluded that a sensible way forward, was to treat a knowledge barrier approach to technology diffusion, as a distinct theory in its own right. The diffusion of technology is reconceptualized in terms of organizational learning, skill development, and knowledge barriers. As knowledge barriers are lowered, diffusion speeds up, and a transition is observed from an early pattern in which the new technology is typically obtained as a service to a later pattern of in-house provision of the technology.
The “rate of adoption is the relative speed with which an innovation is adopted by members of a social system” Rogers (2003). Therefore, throughout the process the FSA will strive to be transparent and engage with the public. Techniques for adoption and integration could include the spherical cow approach in order for maximum understanding for all stakeholders, including the consumer. Data visualisation exploration techniques could be applied (e.g., Mondrian and Manet (Unwin et al., 1996)) to make use of multidimension data. Incorporating data sets such as Open FoodTox into the latest predictive models will increase predictive capacity for chemicals with little or no tox data.
As stated in the KTN Innovation 4.0 Playbook “Technological applications of AI require explicit consideration of ‘explainability’ and trust”. The FSA propose to have data integrity and data capability ambitions to fulfil this.
Data integrity and data capability
The FSA will strive to the following ambitions:
- facilitate data tools.
- enhance computational resources.
- develop and support training and skills in computer science/data.
- build confidence and trust in data usage.
- provide data integrity and data cleaning.
- demonstrate leadership in developing new methodologies towards using the best scientific available tools towards risk assessment.
Transition and Integration
Innovative technologies should be reviewed and evaluated once, prior to integration into the risk assessment/as a chemical testing method, as part of the risk assessment strategies for chemical testing for human health and the environment. Using an evidence-driven approach, to address the data gaps in the risk assessment process, will facilitate the acceptance and validity of these NAMs, as well as pave the way for alternative testing strategies with confidence.
It is hard for new technologies to be accepted because regulations, infrastructure, user practices and maintenance networks are aligned to the existing technology (Geels, 2002). In order for these new technologies to progress, users have to integrate them into their practices, organisations and routines. This involves learning/training, adjustments and ‘domestication’ (Lie and Sørensen, 1996). It is known that links between technical and social elements provide stability leading to sociotechnical change.
The FSA will strive to take into account using the Political, Economic, Sociological, Technological, Legal and Environmental (PESTLE) analysis method towards making strategic decisions. Using guiding principles from the Green Book we can start to analyse the design and use of monitoring and evaluation before, during and after implementation.
Inserting economics values such as Disability-adjusted life years (DALYs), quality- adjusted life year (QALY) Willingness-To-Pay (WTP) and even into chemical space will be key to demonstrate that applying such values would lead to better and more informed decisions. In HSEs Value of a Life Year (VOLY) report it stated that it would have major implications not only for efficiency of government spending but also for equity in population wellbeing.
This COT FSA UK roadmap will pave the way towards acceptance and integration of NAMs into safety and risk assessments from a regulatory perspective and make use of the UK government Research & Development Roadmap which will form part of the wider systems thinking approach.
Integration of these technologies as part of the chemical risk assessment process will be fundamental in the future of human safety i.e. the consumer. This will use the best science available to predict and assess risk more rapidly, accurately, and efficiently (Figure 5).
Figure 5. Paradigm shift to towards the vision of predicting risk more rapidly, accurately and efficiently.
The 7 Steps to Integration & Acceptance
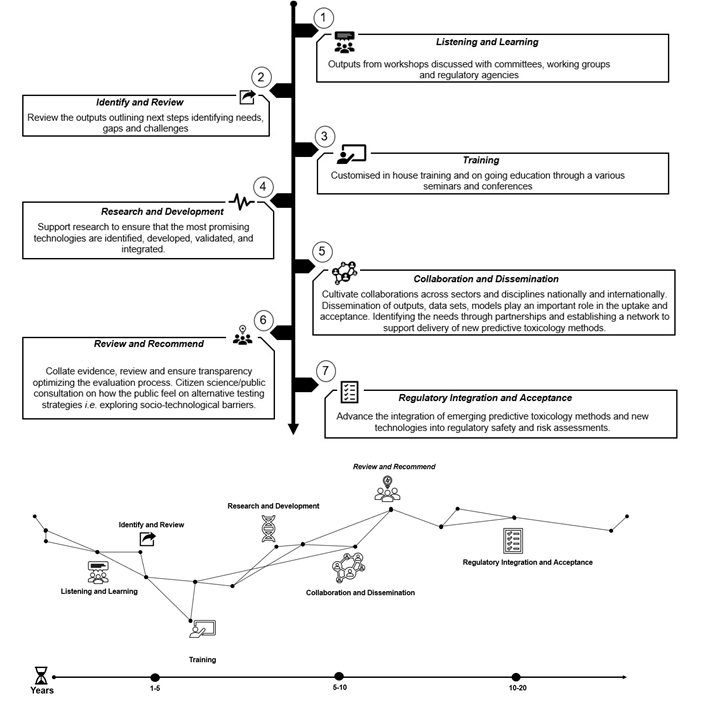
The UK Roadmap comprises of 7 steps to integration and acceptance: (1) Listening and Learning (2) Identify and Review (3) Training (4) Research and Development (5) Collaboration and Dissemination (6) Review and Recommend (7) Regulatory Acceptance and Integration.
Figure 6. The UK Roadmap (NB The process is not linear and the steps in the roadmap diagram are not intended to represent a comprehensive set of activities with a precise timescale but should be taken as an illustration of the broad landscape vision).
Overall objectives of the roadmap are to:
- identify latest available NAMs for optimal risk assessment.
- Learn from other regulatory agencies and beyond.
- Validate through case studies.
- Build confidence in NAMs in the regulatory setting.
- Develop skills and training.
- Implement and integrate NAMs in the regulatory setting.
Listening and Learning
Outputs from workshops organised by the FSA and the COT will be discussed with relevant and appropriate committees, working groups and regulatory agencies for opportunity to comment and provide input.
The FSA will review what other regulatory agencies and industry have done and what still needs to be done.
Identify and Review
Identify available NAMs and what the pros and cons for each are and where their strengths lie.
Formulate the problem space.
Throughout the process continue to review internal and external research and development and how that may impact on the roadmap.
Customised in-house training and ongoing education through various seminars and conferences.
Skill development: building a resilient organization.
From the perspective of FSA and COT we can then suggest that other regulators / cross government would need similar training.
Research and Development
Support and initiate research to ensure that the most promising technologies are identified, developed, validated, and integrated.
Assess the list of NAMs and other NAMs roadmaps.
Funding and Recruitment
Recruitment of Computational Fellow. Recruitment of Tox AI PhD student.
Collaboration and Dissemination
Cultivate collaborations across sectors and disciplines nationally and internationally.
Dissemination of outputs, data sets and models will play an important role in the uptake and acceptance of NAMs. Identifying the acceptance needs through partnerships and establishing a network to support delivery of new predictive toxicology methods.
Network with regulatory agencies and academics in this workspace.
Set up a hub in the FSA and Cross-Government, industry, and academia to disseminate models etc.
Set up a Cross Whitehall NAMs working group so we can exchange information in this area across government departments.
[The FSA have created and lead the Cross Whitehall group on NAMs New Approaches to Chemical Risk Assessment in the Regulatory Space (NACRARS)]
Citizen science and public engagement.
Review and Recommend
Collate evidence, review, and ensure transparency. Optimizing the evaluation process.
Maintain open transparency throughout.
Public consultation on how the public feel on alternative strategies and approaches. Present work throughout to working groups and scientific advisory committees.
Regulatory Integration and Acceptance
Advance the integration of emerging predictive toxicology methods and new technologies into regulatory safety and risk assessments.
Data integrity and data capability.
Integrate the best possible methodologies in risk assessment to predict risk more efficiently, rapidly and accurately.
Virtual laboratories acceptance.
Integration of NAMs in a regulatory setting.
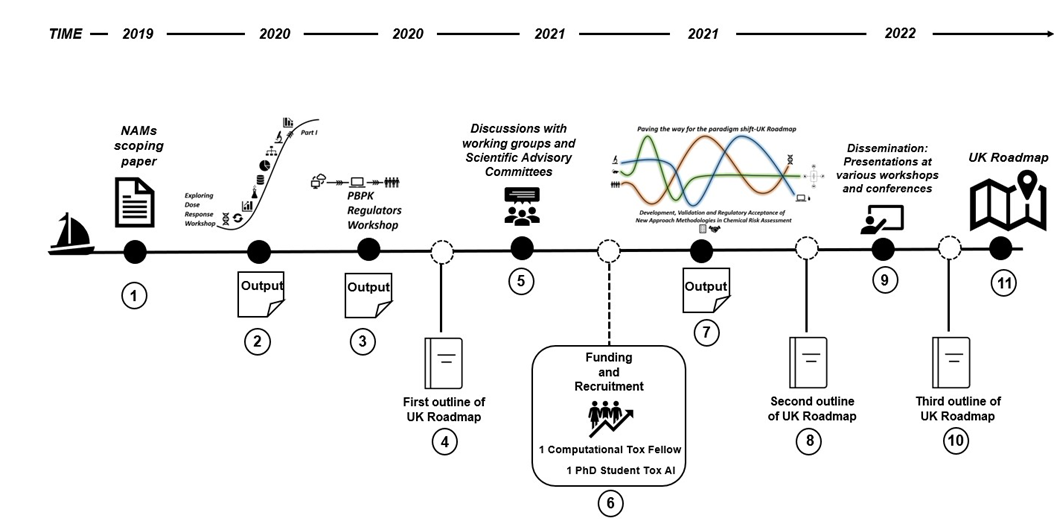
Stepping and (Mile)Stones - How the FSA and COT got here.
Paving the way for a UK Roadmap journey - How the FSA got here:
Stepping and (Mile)Stones - How the FSA and COT got here.
- NAMs scoping paper taken to COT and reviewed.
- Output of the Exploring Dose Response Workshop (March 2020)
- Output of the PBPK Regulators Workshop (December 2020)
- Output of the discussions with working groups and scientific advisory committies (regular reviews)
- First outline of the UK Roadmap (2021)
- Recruitment of a PhD student and computational tox fellow.
- Output of the Paving the way for the paradigm shift-UK Roadmap Development, Validation and Regulatory Acceptance of New Approach Methodologies workshop (October 2021)
- Second outline of the UK Roadmap (2022)
- Dissemination presentations at varoius workshops and conferences
- Third outline of UK Roadmap (2023)
- Finalisation of the UK Roadmap.
Finding Balance
We are now at a pivotal point of finding the balance between in vitro/in vivo/in chemico/in silico data (Figure 7). Traditional methods were the best available methods at the time and these included the use of animals and it is important to recognise that these have served us well. However, we have now entered a new era that requires us to adopt new technologies to ensure that we continue to use the best methodologies available to make our risk assessment process as reliable and relevant as possible. This will require an integrated workflow approach (Figure 2).
Figure 7. Finding the balance from Mice to Mouse.
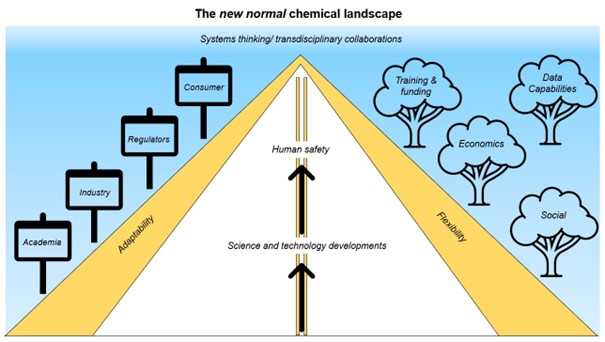
Future Visions: The new normal chemical landscape
As the chemical cosmos is likely to increase, to navigate the future complexities of it, especially with respect to risk assessment, will require the best methodologies available.
The future of food safety assessment of chemicals depends on our adaptability and flexibility whilst using the best scientific methodologies and strategies available in order to respond to the accelerating developments in science and technology.
The future chemical safety landscape will require systems thinking and transdisciplinary collaborations. It will not only require traditional themes but economics, social, training and funding in new areas (such as data capabilities) to focus on gains and benefits these new technologies offer to protect the public.
The vision is to be able to predict risk more rapidly, accurately and efficiently.
Figure 8. The new normal chemical landscape.
References
Attewell, P., 1992. Technology diffusion and organizational learning: The case of business computing. Organization science, 3(1), pp.1-19.
Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) Committee on Toxicity | Committee on Toxicity (food.gov.uk)
Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) Scoping paper Environmental, health and safety alternative testing strategies: Development of methods for potency estimation TOX/2019/70 Developing methods for potency estimation (food.gov.uk).
Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) Minutes December Meeting (2019) COT December 2019 Final Minutes (food.gov.uk).
Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) Exploring Dose Response Workshop Report (2020) Exploring Dose Response Workshop Report (food.gov.uk).
Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) PBPK for Regulators Workshop Summary (2020) Blank style sheet for COT Papers 2014 (food.gov.uk).
Cronin, M.T., Madden, J.C., Yang, C. and Worth, A.P., 2019. Unlocking the potential of in silico chemical safety assessment–A report on a cross-sector symposium on current opportunities and future challenges. Computational Toxicology, 10, pp.38-43
Department for Environment, Food & Rural Affairs and Government Office for Science research programme launched to inform Defra policy making (2019) Science research programme launched to inform Defra policy making - GOV.UK (www.gov.uk).
Department Health Social Care- Better, broader, safer: using health data for research and analysis Independent Report Better, broader, safer: using health data for research and analysis - GOV.UK (www.gov.uk).
Department for Business, Energy and Industrial Strategy (BEIS) white paper on Regulation for the Fourth Industrial Revolution (2019) Regulation for the Fourth Industrial Revolution: White Paper (publishing.service.gov.uk).
Department for Business, Energy and Industrial Strategy (BEIS) Report-The use of emerging technologies for regulation (2020) The Use of Emerging Technologies for Regulation (publishing.service.gov.uk).
Environment Protecting Agency-Accelerating the Pace of Chemical Risk Assessment (APCRA) Accelerating the Pace of Chemical Risk Assessment (APCRA) | US EPA
European Food Safety Authority Open Food Tox. Chemical Hazards Database (OpenFoodTox) | EFSA (europa.eu).
Díaz, S., Demissew, S., Carabias, J., Joly, C., Lonsdale, M., Ash, N., Larigauderie, A., Adhikari, J.R., Arico, S., Báldi, A. and Bartuska, A., 2015. The IPBES Conceptual Framework—connecting nature and people. Current opinion in environmental sustainability, 14, pp.1-16.
EU FOOD 2030 Pathways for Action, Food Systems and Data (2018) Language selection | European Commission (europa.eu).
Food Standards Agency (FSA). Homepage | Food Standards Agency
Food Standards Agency Incidents Food incidents, product withdrawals and recalls | Food Standards Agency.
Food Standards Agency Chief Scientist Data Science Report (2017) Chief Scientific Adviser's Science Report (food.gov.uk).
Food Standards Strategy Our strategy | Food Standards Agency.
Geels, F.W., 2002. Technological transitions as evolutionary reconfiguration processes: a multi-level perspective and a case-study. Research policy, 31(8-9), pp.1257-1274.
Global Summit on Regulatory Science (GSRS17) was Emerging Technologies for Food and Drug Safety Report (2017). 2022-12-16 08:12 | Archive of FDA (pagefreezer.com).
Goel, V. and Pirolli, P., 1992. The structure of design problem spaces. Cognitive science, 16(3), pp.395-429.
Goel, V. and Pirolli, P., 1989. Motivating the notion of generic design within information-processing theory: The design problem space. AI magazine, 10(1), pp.19-19.
Government Food Strategy Government food strategy - GOV.UK (www.gov.uk).
Government -The Green Book: appraisal and evaluation in central government The Green Book: appraisal and evaluation in central government - GOV.UK (www.gov.uk).
Government for Science Rebuilding a resilient Britain Rebuilding a resilient Britain - GOV.UK (www.gov.uk).
Government for Science Rebuilding a Resilient Britain: Data and Evaluation Areas of Research Interest across Government Data_and_Evaluation_ARI_summary_paper.pdf (publishing.service.gov.uk).
Hartung, T., 2010. Lessons learned from alternative methods and their validation for a new toxicology in the 21st century. Journal of Toxicology and Environmental Health, Part B, 13(2-4), pp.277-290.
Judson, R., Richard, A., Dix, D.J., Houck, K., Martin, M., Kavlock, R., Dellarco, V., Henry, T., Holderman, T., Sayre, P. and Tan, S., 2009. The toxicity data landscape for environmental chemicals. Environmental health perspectives, 117(5), pp.685-695.
Kavlock, R., Chandler, K., Houck, K., Hunter, S., Judson, R., Kleinstreuer, N., Knudsen, T., Martin, M., Padilla, S., Reif, D. and Richard, A., 2012. Update on EPA’s ToxCast program: providing high throughput decision support tools for chemical risk management. Chemical research in toxicology, 25(7), pp.1287-1302.
Lie, M. and Sørensen, K.H. eds., 1996. Making technology our own?: domesticating technology into everyday life. Scandinavian University Press.
Malloy, T., Zaunbrecher, V., Beryt, E., Judson, R., Tice, R., Allard, P., Blake, A., Cote, I., Godwin, H., Heine, L. and Kerzic, P., 2017. Advancing alternatives analysis: the role of predictive toxicology in selecting safer chemical products and processes. Integrated environmental assessment and management, 13(5), pp.915-925.
National Centre for the Replacement, Refinement and Reduction (NC3Rs). The 3Rs | NC3Rs.
Newell, A., Yost, G.R., Laird, J.E., Rosenbloom, P.S. and Altmann, E., 1993. Formulating the problem space computational model. In The Soar papers (vol. II) research on integrated intelligence (pp. 1321-1359).
Pain, G., Hickey, G., Mondou, M., Crump, D., Hecker, M., Basu, N. and Maguire, S., 2020. Drivers of and Obstacles to the Adoption of Toxicogenomics for Chemical Risk Assessment: Insights from Social Science Perspectives. Environmental health perspectives, 128(10), p.105002.
Rogers, E.M. (2003). Diffusion of innovations, 5th Ed. New York, NY.
Royal Society of Chemistry Drivers and scope for a UK chemicals framework (2021) rsc-uk-chemical-framework-drivers-scope-2020.pdf.
Russell, W.M.S. and Burch, R.L., 1959. The principles of humane experimental technique. Methuen.
Simon, H.A., 2019. The sciences of the artificial. MIT press. Science Council Science Council | Science Council (food.gov.uk).
Science Council Working Group on Data Usage and Digital Technology Report (2020) Final Report from the Science Council Working Group on Data Usage and Digital Technology (food.gov.uk).
Slikker Jr, W., de Souza Lima, T.A., Archella, D., de Silva Junior, J.B., Barton- Maclaren, T., Bo, L., Buvinich, D., Chaudhry, Q., Chuan, P., Deluyker, H. and
Domselaar, G., 2018. Emerging technologies for food and drug safety. Regulatory Toxicology and Pharmacology, 98, pp.115-128.
Solomon, K.R., Wilks, M.F., Bachman, A., Boobis, A., Moretto, A., Pastoor, T.P., Phillips, R. and Embry, M.R., 2016. Problem formulation for risk assessment of combined exposures to chemicals and other stressors in humans. Critical Reviews in Toxicology, 46(10), pp.835-844.
Sturla, S.J., Boobis, A.R., FitzGerald, R.E., Hoeng, J., Kavlock, R.J., Schirmer, K., Whelan, M., Wilks, M.F. and Peitsch, M.C., 2014. Systems toxicology: from basic research to risk assessment. Chemical research in toxicology, 27(3), pp.314-329.
Synthesis and Integration of Epidemiological and Toxicological Evidence Subgroup (SETE) -Draft report on the synthesis and integration of epidemiological and toxicological evidence in risk assessments (2021).
TOX-2021-37 Draft Report of the SETE subgroup of the COT and COC
UK GOV Policy Paper- Genome UK: the future of healthcare (2020). Genome UK: the future of healthcare - GOV.UK (www.gov.uk).
UK GOV Global Britain in a competitive age The Integrated Review of Security, Defence, Development and Foreign Policy Global Britain in a Competitive Age the Integrated Review of Security Defence Development and Foreign Policy.pdf.
UK GOV UK Research and Development Roadmap UK Research and Development Roadmap (publishing.service.gov.uk).
UKRI Non-animal technologies in the UK: a roadmap, strategy and vision Non-animal technologies in the UK: a roadmap, strategy and vision – UKRI.
UKRI Strategy 2022-2027 Our strategy 2022 to 2027 – UKRI.
Unwin, A., Hawkins, G., Hofmann, H. and Siegl, B., 1996. Interactive graphics for data sets with missing values—MANET. Journal of Computational and Graphical Statistics, 5(2), pp.113-122.
Wang, Z., Walker, G.W., Muir, D.C. and Nagatani-Yoshida, K., 2020. Toward a global understanding of chemical pollution: a first comprehensive analysis of national and regional chemical inventories. Environmental science & technology, 54(5), pp.2575-2584.
Worth, A.P., 2019. The future of in silico chemical safety… and beyond. Computational Toxicology, (10) pp 60-62.
Abbreviations
|
3Rs |
Replacement, Reduction and Refinement |
|
4IR |
4th industrial revolution |
|
AI |
Artificial Intelligence |
|
AOPs |
Adverse outcome pathways |
|
ARIs |
Areas of Research Interest |
|
COC |
Committee on Carcinogenicity |
|
COT |
Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment |
|
DALYs |
Disability-adjusted life years |
|
FSA |
Food Standards Agency |
|
HTS |
High Throughput Screening |
|
IATAs |
Integrated approaches to testing and assessment |
|
NC3Rs |
National Centre for the Replacement, Refinement and Reduction of Animal Research |
|
NAMs |
New Approach Methodologies |
|
PBPK |
Physiologically based pharmacokinetics |
|
PODs |
Points of departure |
|
SETE |
Synthesis and Integration of Epidemiological and Toxicological Evidence Subgroup |
|
SERD |
Science, Evidence and Research Division |
|
UKHSA |
UK Health Security Agency |
|
QALY |
Quality Adjusted Life Year |
|
QSAR |
Quantitative structure Activity Relationships |
|
VOLY |
Value of Life Year |
Technical information
21st century toxicology (Tox 21) refers to ‘the transformation underway in the tools and approaches used to evaluate chemical substances for possible effects on human health’. National Research Council, 2007. Toxicity testing in the 21st century: a vision and a strategy.
National Academies Press. Toxicity testing in the 21st century: a vision and a strategy. National Academies Press: Toxicity Testing in the 21st Century: A Vision and a Strategy |The National Academies Press.
disability-adjusted life year (DALY) is a measure of overall disease burden, expressed as the number of years lost due to ill-health, disability or early death. It was developed in the 1990s as a way of comparing the overall health and life expectancy of different countries.
High throughput screening (HTS): is a method for scientific experimentation especially used in drug discovery and relevant to the fields of biology and chemistry. Using robotics, data processing/control software, liquid handling devices, and sensitive detectors, high-throughput screening allows a researcher to quickly conduct millions of chemical, genetic, or pharmacological tests. Through this process one can rapidly identify active compounds, antibodies, or genes that modulate a particular biomolecular pathway. The results of these experiments provide starting points for drug design and for understanding the noninteraction or role of a particular location.
Integrated approaches to testing and assessment (IATAs): provide a means by which all relevant and reliable information about a chemical is used to answer a defined hazard characterization question. Information considered can include toxicity data, computational model predictions, exposure routes, use cases, and production volumes. This information is used to characterize outcomes that can inform regulatory decision-making. (Integrated Approaches to Testing and Assessment)
In silico: one performed on computer or via computer simulation.
MANET : is for exploring data, whether raw data, transformed data or model residuals. MANET provides a range of graphical tools specially designed for studying multivariate features. MANET useful for gaining insights into the structure and relationships of their data sets.
Mondrian: is a general-purpose statistical data-visualization system.
Omics: are various disciplines in biology whose names end in the suffix -omics, such as genomics, proteomics, metabolomics, metagenomics and transcriptomics.
Political, Economic, Sociological, Technological, Legal and Environmental (PESTLE) analysis studies the key external factors that influence an organisation. It can be used in a range of different scenarios, and can guide people professionals and senior managers in strategic decision-making.
quality-adjusted life year (QALY) is a generic measure of disease burden, including both the quality and the quantity of life live.
Rebuilding a Resilient Britain programme: builds on work to develop government science capability and the external evidence base to support policy development.
This report sets out more analysis relating to data and evaluation. It examines existing questions to identify cross-cutting themes, and to provide a platform for engagement between government departments and academics to consider medium and long-term questions.
Spherical cow: is a humorous metaphor for highly simplified scientific models of complex real-life phenomena.
The Fourth Industrial Revolution (4IR): is the fourth major industrial era since the initial Industrial Revolution of the 18th century. It is characterized by a fusion of technologies that is blurring the lines between the physical, digital and biological spheres, collectively referred to as cyber-physical systems.
Value of Life Year (VOLY) values the impact of risks to the length of life.
Zettabytes: are 1,000,000,000,000,000,000,000 bytes. Zettabyte is approximately equal to a thousand Exabytes, a billion Terabytes, or a trillion Gigabytes.
Acknowledgments
The FSA and COT would like to thank everyone who contributed to the roadmap and the participation of attendees of the workshops that have helped shaped the roadmap.
More information
FSA Website: Homepage | Food Standards Agency
COT Website: Committee on Toxicity | Committee on Toxicity (food.gov.uk)
NAMs contact email: nams@food.gov.uk